This month, the approval of an RNA-based vaccine for COVID-19 took another step forward with the endorsement last week by Japanese public health authorities of another form of RNA that can make copies of itself inside cells. This time, however, it is the first ‘self-amplifying’ RNA (saRNA) granted full regulatory approval anywhere in the world.
The new vaccine platform could provide defenses ranging from various infectious diseases and conceivably possibly other major health concerns. And because it could be used at a lower dose, it might have fewer side effects than other messenger RNA (mRNA) treatments have.
Global research efforts to search for new vaccines have been ramped up with the availability of CRISPR. But where we are now is the culmination of vaccine history.
Putting this new saRNA vaccine in context
Most of us are familiar with vaccines that benefit us humans but are less aware of the role of vaccines for veterinary purposes also relevant in the development of vaccines for humans. These outnumber human options, partly due to animal vaccines having fewer regulatory requirements than human vaccines, resulting in less time and money involved in the creation and production of animal vaccines.
In the past, the human vaccine development process generally took 10 to 15 years, whereas the animal vaccine process only took an average of 5 to 7 years to produce. The importance of veterinary-related vaccines is in domestic animals and wildlife prevention against rabies, distemper, parvovirus, and equine influenza, to mention only a few. That there are benefits to human and animal health is core to the One Health approach, which also integrates plant and environmental health.
As we all know, the modern vaccine era began, in 1796, when Edward Jenner developed the smallpox vaccine, The 1800s witnessed many newcomers, including the first cholera vaccine in the early 1800s (a safe and effective one was not developed till deep in the next century), and the first rabies vaccine in the 1880s; the typhoid vaccine in the late 1800s was also just the initial efforts that were later improved.
Major achievements in the 1900s included the development of the diphtheria vaccine in the 1920s, tuberculosis and yellow fever vaccines in the 1930s, the pertussis vaccine in the 1940s, the polio vaccine in the 1950s, the measles vaccine in the 1960s, and the hepatitis B vaccine in the 1980s.
In our current century, the pace of vaccine discovery and innovation accelerated with the introduction of new technologies and advancements.
Examples include the rotavirus vaccine, the human papillomavirus (HPV) vaccine, the varicella vaccine, the pneumococcal conjugate vaccine, the dengue and Ebola vaccines, and new vaccine research in such growing infectious disease threats including chikungunya vaccine in the process of certification in the United States.
Veterinary vaccine technologies include gene-deleted marker vaccines, virus-like particles, recombinant modified live virus vaccines, chimeric vaccines, and a wide variety of novel adjuvants. Human vaccine researchers have and continue to learn and use what is done for animals to benefit humans. Research and development of veterinary vaccines enable efficient production of food animals to feed the human population, and greatly reduce the need for antibiotics to treat food and companion animals.
This is not to suggest that extensive research efforts have always resulted in success: HIV/AIDS is an example of huge research efforts and investment that has not resulted in a vaccine.
And of course, the COVID-19 pandemic catalyzed the expedited development of multiple vaccines targeting the SARS-CoV-2 virus, with several vaccines having received authorization for mass immunization.
The emergence of messenger RNA vaccines represented a groundbreaking innovation in vaccine technology. mRNA vaccines work by providing the genetic instructions for cells to produce a viral antigen, triggering an immune response.
The versatility of an mRNA platform has shown it is a pathway for the rapid design and production of new and improved vaccines. And the possibility of therapeutic vaccines, for say cancer or shingles, could be big in the future.
But now out of the mRNA platform yet another form of vaccine, the self-amplifying saRNA vaccine has emerged.
How “saRNA”, the newest entry into dealing with COVID, works
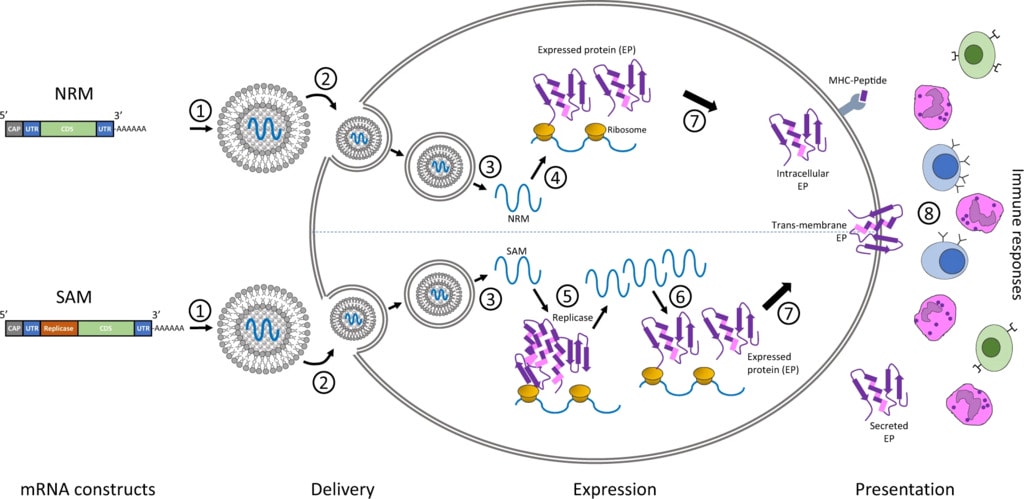
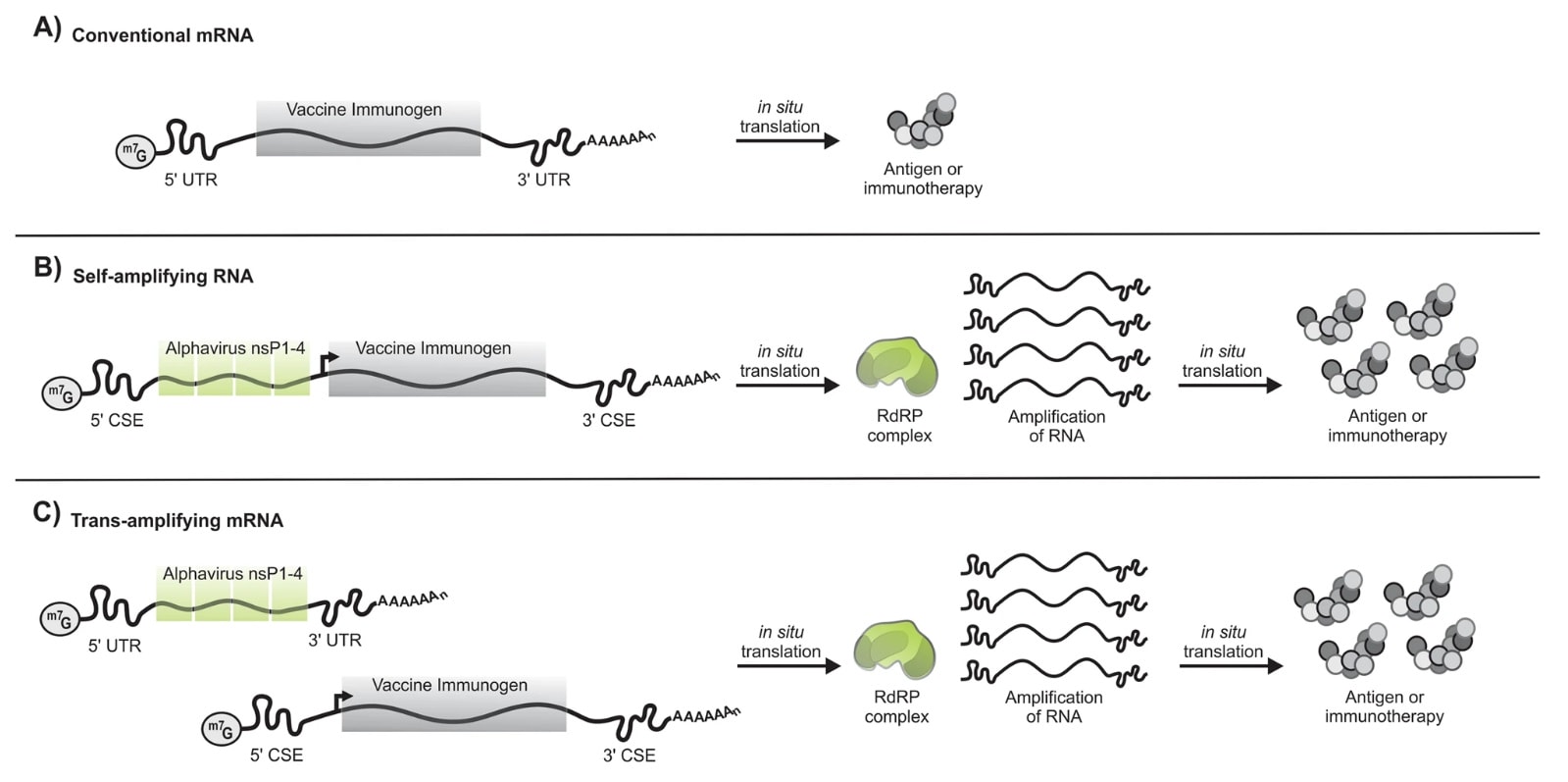
Conventional mRNA-based COVID-19 consists mainly of the genetic instructions for a viral protein that are surrounded by regulatory sequences. A cell’s machinery produces the protein for as long as these instructions persist, and that protein — known as an antigen — stimulates an immune response.
By contrast, saRNA goes a step further by integrating the genes needed for the replication and synthesis of the antigen-encoding RNA, effectively establishing a biological printing press for fabricating the vaccine inside cells.
Approved by Japanese regulatory authorities, “ARCT-154” was developed by Arcturus Therapeutics in San Diego, California, and its partner CSL headquartered in Melbourne, Australia. This new saRNA vaccine platform is not simply a variation on conventional mRNA shots, “but in practice, it’s really not”, says Anna Blakney, a bioengineer who studies the technology at the University of British Columbia in Vancouver, Canada. “saRNA is a totally different beast.”
Importantly, ARCT-154 requires one-tenth to one-sixth as much vaccine per person as other RNA-based COVID-19 boosters. Reducing the amount of vaccine administered in each injection should result in lower production costs.
Because of its virus-like nature, saRNA interacts with the immune system in distinctive ways that could prove beneficial across a range of disease scenarios. When it comes to preventing infections, for instance, its self-amplifying capabilities could enable the use of lower vaccine doses.
That said, a saRNA vaccine does have some potential downsides.
For example, because of added genetic instructions requiring longer sequences — typically at least three times longer than conventional mRNA shots — it adds complexity to the manufacturing process.
The new saRNA stimulates beneficial immune-signaling but excessive stimulation can have the vaccine result in blocking RNA replication. The challenge is in determining the optimal dose to get the balance right and this has been tried for decades by the biotechs but has proved to be an obstacle which hadn’t been overcome.
With the approval of the saRNA platform in this instance in Japan, it will now go beyond clinical trials, and we will learn how viable this approach is.
What About the Future?
The International Vaccine Institute (IVI) (established in 1997 as an initiative of the United Nations Development Programme), based in South Korea, is engaged in every step of the vaccine value chain, from discovery to development, delivery, and capacity building. It will be looking at other saRNA vaccine candidates currently in clinical trials.
With the approval for ARCT-154 secured in Japan, its developers are now seeking authorization in Europe; a regulatory decision is expected next year.
Further, “This will hopefully begin to put a nail in the coffin of the idea that self-amplifying RNA is not a viable platform,” says Corey Casper, president and chief executive of the Access to Advanced Health Institute in Seattle, Washington.
And more research for saRNA platforms is being funded: Last August, the Coalition for Epidemic Preparedness Innovations (CEPI) announced that it was committed to providing up to $3.6 million for the development of self-amplifying saRNA platforms. “In the case of saRNA vaccines, genetic information from a particular group of viruses is incorporated into the saRNA together with the antigen of interest. The genetic information from the virus programmes the host cell to generate multiple copies of the saRNA, hence the term self-amplification.”
It is feasible to take a wider view, that of future vaccine candidates against unknown pathogenic threats, which is referred to as ‘Disease X’.
According to CEPI, “the threat of Disease X infecting the human population, and spreading quickly around the world, is greater than ever before”. And as mentioned, there is the potential for vaccine research to address therapeutic aspects, such as shingles, the flu, and cancer. Wouldn’t that be wonderful if we had them?
Editor’s Note: The opinions expressed here by the authors are their own, not those of Impakter.com — Featured Photo Credit: screenshot – from video interview of Arcturus Therapeutics CEO speaking of their new saRNA vaccine