Worldwide we have seen and regularly reported that there have been significant increases in known illnesses such as diabetes, cancer, multiple sclerosis, and schizophrenia – and along with that increase, there have been significant advances in knowledge and how to deal with them. And lately new research tools have become available, in particular genetic research: By extracting DNA from ancient bones and teeth, a picture of how past diseases evolved through time can be obtained together with a better understanding on how to address them.
As the global prevalence of rare diseases is increasing, this could be especially useful as this is an area where a lot less research has been carried out so far. Yet rare diseases affect between 3.5% and nearly 6% of the worldwide population at any point in time, which adds up to a substantial number. Recent research has conservatively estimated the affected people at 300 million worldwide or 4% of an estimated world population of 7.5 billion (the number used until now by EURORDIS and Rare Diseases International).
So far, more than 6,800 rare diseases have been identified, of which 72% are genetic. Seventy percent of rare genetic disorders start in childhood. A genetic disorder is caused by a change in a gene or group of genes that are present from birth. Thus, research into such rare diseases is valuable for existing and especially for future generations.
All of this underscores that our current knowledge of the vast universe of possible diseases and their genesis in our genes is comparatively limited, but there is new scientific research that can make a huge difference.
Learning from the distant past: Genetic research into the DNA of ancient populations give insight for us today
Analysis of the genetic makeup of those who lived thousands of years ago is beginning to help to better understand factors that contribute to genetic resistance to diseases and the environment. Research is garnering interest around the world in analyzing genetic resistance in ancient populations. From such information, it is hoped that ways can be found to develop targeted approaches for managing and preventing diseases in populations today.
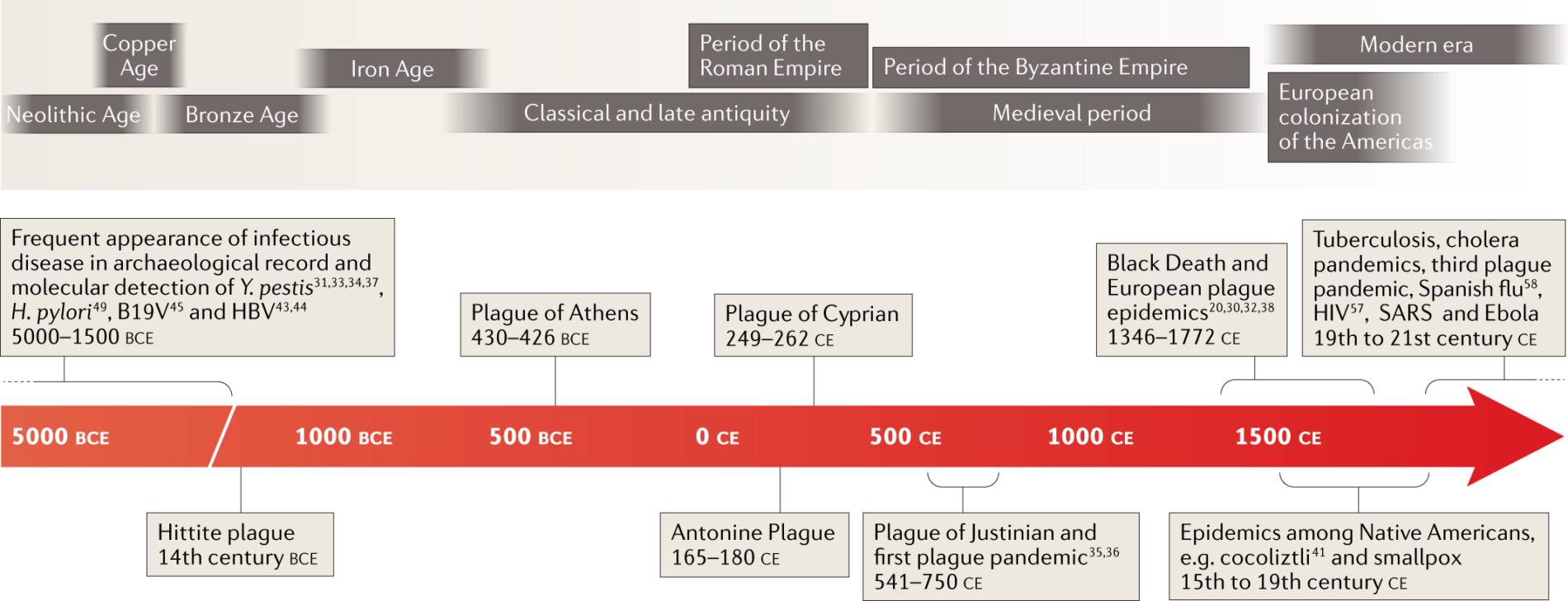
An article published in Nature in 2019, titled Ancient pathogen genomics as an emerging tool for infectious disease research, notes that “Although most of their causative agents still remain speculative, robust molecular methods coupled with archaeological and historical data can confidently demonstrate the involvement of certain pathogens in these episodes.” And an explanatory table is provided to illustrate the historical scope of the research:
The 5,000 Ancient Human Genomes Project built a data set of ancient human genomes, analyzing bones and teeth made available through a scientific partnership, the results of which are described in four articles in Nature.
The team of experts working on this project is impressive: The research was led by experts from the University of Copenhagen with contributions from around 175 researchers from universities and museums in the U.K., the U.S., Germany, Australia, Sweden, Denmark, Norway, France, Poland, Switzerland, Armenia, Ukraine, Russia, Kazakhstan, and Italy. They included a broad range of expertise, from archaeologists, ancient DNA researchers, and evolutionary biologists to medical professionals, infectious disease researchers, and epidemiologists.
Five thousand specimens were from the Mesolithic, Neolithic, Bronze Ages, and the later Iron Age, Viking period, into the Middle Ages. The oldest genome was from an individual who lived approximately 34,000 years ago.
The goal was to trace the effect of ancient diseases through time, thus identifying clues to so-called “modern medical mysteries”.
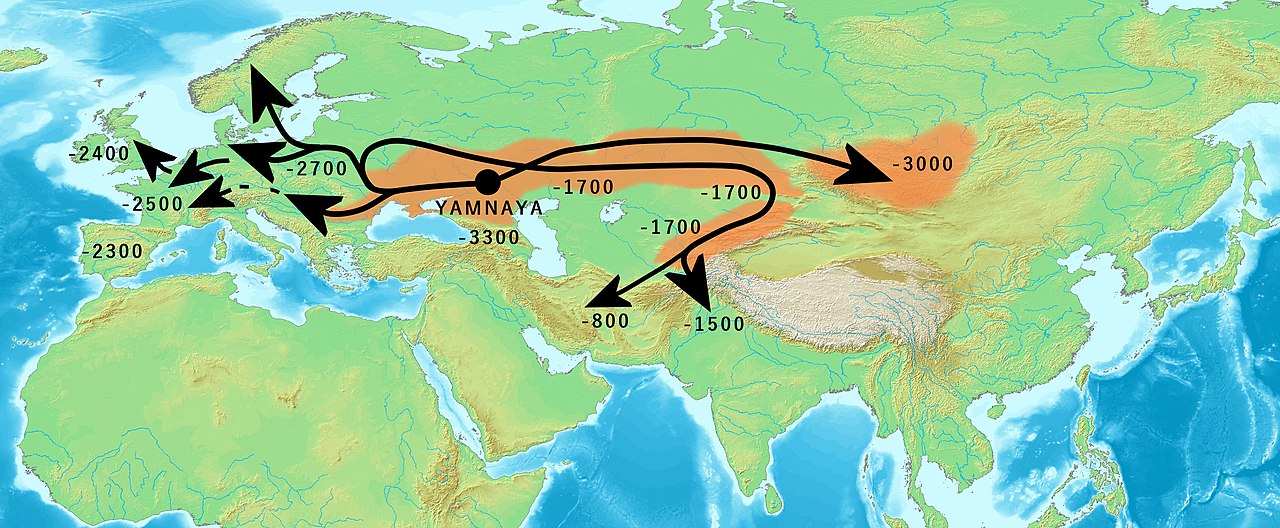
A case in point was the study of a major migration of pastoralist herders known as the Yamnaya people spanning parts of what are now Ukraine, South-West Russia, and West Kazakhstan.
The Francis Crick Institute carried out another interesting study identifying potential new ways to address lung cancer, also published in Nature in April 2023. It found that ancient viruses remnants in human DNA, possibly thousands or even millions of years old, could help fight cancer, specifically, “dormant relics of old cells can be activated by cancerous cells…can then inadvertently help the immune system target and attack the tumor.” And assuredly there will be others going forward.
A complicated process…
Genetic research, despite technological advances, is not without its problems. Extracting DNA from ancient bones and teeth, however, is an exacting process and one not without risk. It requires a series of steps, any one of which can affect the reliability of the final results.
Briefly, it starts with the condition of the sample. There are risks associated with contamination during DNA handling and on the type of tests being taken. For example, trait tests for a single gene are likely to provide more reliable results.
What we do know is that over the last 5-10 years, technological advances have improved the process, and we can expect that it will continue to do so in the future.
Simply put, ancient genetic variants associated with resistance to specific pathogens can help design more effective vaccines and treatment strategies.
The potential benefits go well beyond, such as learning about how ancients coped with their environment, climate change, exposure to natural toxins, and even food insecurity. The genetic signatures of those who survived thousands of years ago in diverse ecological settings can identify genetic adaptations that allowed them to withstand environmental pressures at that time. And potentially, if our own genetic makeup could be likewise adapted, it would allow us better deal with our world today.
…And such genetic research can benefit animals
There is more. Research on animal genetic resistance in past eras can identify factors that influenced their health and survival as it can do for humans.
Genetic adaptations that enabled animals to thrive in challenging environments and resist infectious diseases could thus be identified. In turn, this would help provide veterinary medicine and animal husbandry with revised or new strategies to enhance the health and welfare of domesticated animals.
Human and animal interactions: Lessons from the past
Further, genetic traits so identified can be harnessed to improve animal breeding practices to deal with disease resistance. and other factors in both livestock and companion animals.
And then there is this: Genetic resistance in ancient populations can tell us about the impact of cultural practices and human-animal interactions on genetic diversity and disease resistance, such as the effects of domestication, animal husbandry, and zoonotic disease transmission.
The promise of ancient genetic research in the future
The results would enrich our understanding of the genetic consequences of human-animal coexistence, and the risks of zoonotic disease emergence. We should not forget the insights gained from the One Health approach that pulls together humans, animals and the environment into an organic whole.
Whether using new research tools to better grapple with the most common afflictions of today, or to gain insight into the 6,000 rare diseases that affect hundreds of millions of people, many of whom are children, there is every reason to believe we are on the verge of having better health for all.
Editor’s Note: The opinions expressed here by the authors are their own, not those of Impakter.com — Featured Photo: Disease in Ancient Egypt: An image of a blind harpist adorns the walls of the tomb of Nakht and his wife Tawy. Trachoma, an infectious eye disease, was common in ancient Egypt and remains a leading cause of blindness today. Source: flickr (used in Gavi.org article about ancient diseases)