Yesterday, the Financial Times reported that Novavax, a US-based pharmaceutical firm, had developed a type of vaccine against Covid using techniques that people are more familiar with rather than the groundbreaking mRNA or viral vectors developed by Pfizer or Moderna – making it both easier to distribute as it doesn’t require special refrigeration and easier to produce, as it can take advantage of existing production facilities everywhere, starting with India, the world’s powerhouse for drug production. The new Novavax vaccine is up for approval from the European drug regulator, possibly as soon as next week.
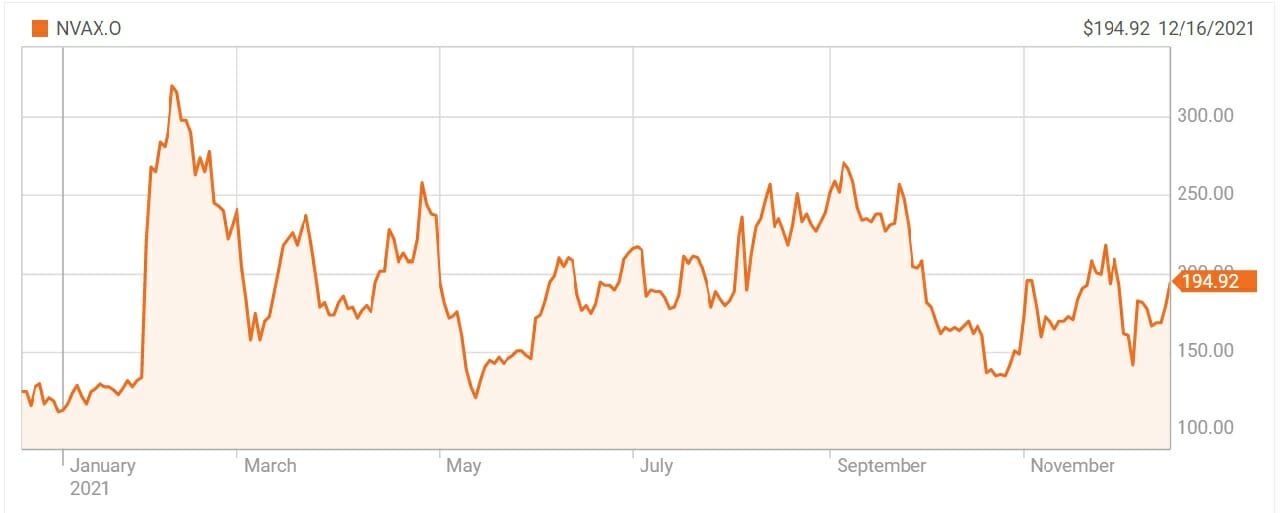
As soon as the news was out, the shares of the US drugmaker rose by 7 percent:
Novavax, an American biotechnology company founded over ten years ago and based in Gaithersburg, Maryland, produces vaccine candidates to respond to both known and newly emerging diseases, using a recombinant nanoparticle vaccine technology. The company’s product pipeline focuses on a range of infectious diseases with various vaccine candidates in clinical development, including vaccines for the Ebola virus, seasonal influenza and pandemic influenza.
What made the news is NVX-CoV2373, Novavax’s new vaccine for SARS-CoV-2, developed in partnership with the Coalition for Epidemic Preparedness Innovations (CEPI) that is providing Novavax up to $388 million in funding for the development and manufacturing of its coronavirus vaccine candidate. In addition, the company was awarded US$1.6 billion from the American government on July 7, 2020 as part of the “Operation Warp Speed” to cover the testing, commercialization and production of a potential coronavirus vaccine in the United States.
Novovax moved fast and released several phase-3 trials results showing first in January 2021 that it has 89% efficacy against Covid-19 and next, in June 2021, that it had an overall 90.4% efficacy in Phase-3 trials in both the U.S and Mexico; and it indicated in relation to the latter that its vaccine likely also provided strong immunity against new variants.
Novavax immediately began its round of applications for emergency use, starting in the US and UK but also working with governments around the world. On May 22, 2021, Novavax and Moderna announced a deal with the South Korean government to manufacture their COVID-19 vaccines. The vaccine is also being co-developed with CEPI in India under the name Covovax.
On September 6, 2021, there was a breakthrough in Japan: Novavax and Takeda Pharmaceutical Co (4502.T) reported that the Japanese government agreed to purchase 150 million doses of the COVID-19 vaccine it will produce using Novavax’s formula. Takeda is Japan’s biggest drugmaker, will make the vaccine domestically and plans to distribute it in early 2022, pending approval from regulators. Meanwhile, the TAK-019 vaccine was undergoing clinical trials in Japan.
On November 1, 2021, Novavax got its first official authorization from a state regulator: Novavax and the Serum Institute of India (SII) announced that the National Agency of Drug and Food Control of Indonesia, or Badan Peng was Obat dan Makanan (Badan POM), had granted emergency use authorization for Novavax’ Covid vaccine with Matrix-M adjuvant. It will be manufactured by SII in India and marketed by SII in Indonesia under the brand name COVOVAX™.
On December 1, 2021, Novavax announced it could begin commercial manufacturing of a COVID-19 vaccine tailored for the Omicron coronavirus variant in January next year, while it tests whether or not its current vaccine works against the variant.
And now Novovax’s vaccine is up for approval by EMA, the European drug regulator, probably by next week. A World Health Organization approval could come once the health body issued its own emergency use listing or if the EMA gave it a conditional marketing authorization, the Financial Times said, citing people familiar with the matter. Novovax is confident that approval is on the horizon, and that could be indeed a game-changer in the battle against Covid.
The reason this is potentially so important is simple: The Novovax vaccine does not require refrigeration and is thus easy to distribute; moreover, it can be produced using available manufacturing facilities around the world. And that means it could be made quickly available to low- and middle-income countries, solving what is by far the greatest challenge we have facing Covid: The possibility that, if the virus is not controlled, it will simply continue to evolve, producing dangerous and/or highly contagious variants à la Omicron.
And finally, cherry on the cake, the Novovax vaccine is more in line with traditional vaccines and does not rely on mRNA techniques that throw fear in so many people. The fear that such vaccines might cause genetic modifications is what drives so many hesitant people in the arms of no-vaxxers. But with a more traditional vaccine, the fear disappears and we can expect even more people willing and lining up to be vaccinated.
With this news, we could indeed be seeing the light at the end of the tunnel and a return to normalcy. Stay tuned for further developments.
Editor’s Note: The opinions expressed here by Impakter.com columnists are their own, not those of Impakter.com. — In the Featured Photo: recombinant nanoparticle Source: Novovax explanation of its vaccine technology, using recombinant nanoparticle.