There is light at the end of the tunnel as the UK, armed with COVID-19 vaccines, embarks on its mission to vaccinate and immunize against the coronavirus. In early December the UK became the first country to approve administration of the Pfizer/BioNTech vaccine; in its first week of deployment, over 130,000 people were vaccinated. The highly efficient vaccine delivers 95% protection from COVID-19 and marks a historic moment where “the searchlights of science” pick out the “invisible enemy” of coronavirus, as Boris Johnson put it at a recent press conference.
The UK government has already ordered 40 million doses of the Pfizer/BioNTech vaccine – enough to immunize 20 million people. Hedging their bets, the UK has secured access to a total of 357 million doses of seven different vaccines. Other promising vaccines with over 90% effectiveness include ones from Moderna and another from a joint venture between Oxford University and AstraZeneca.
[O]ver 137,000 people have already received the first dose of the coronavirus vaccine.
This is just the start and we will steadily expand our vaccination programme – ultimately helping everyone get back to normal life.
– Matt Hancock, Health and Social Care Secretary.
The race for a COVID-19 vaccine has brought pharmaceutical and research companies into a frenzy of fierce competition. To date, there are over 198 vaccines being vetted by companies from all corners of the world. Three of the most developed western vaccines – Oxford/AstraZeneca, Pfizer/BioNTech and Moderna – have the production capacities for 5.3 billion doses in 2021, enough to vaccinate between 2.6 billion and 3.2 billion people (depending on the dosage administered).
Propelling the notion of a race, Russia became the first country to approve a COVID-19 vaccine this summer, its own Sputnik V, even before it had passed all trial stages. Since then, Moscow has shipped the vaccine to various countries across Latin America and the Middle East as part of clinical trials. According to the Russian Health Minister Mikhail Murashko, 100,000 people have already been vaccinated whilst current Sputnik V trials involve a total of 40,000 people.
These numbers will skyrocket as President Putin’s mass vaccination project takes effect and the production of 2 million doses nears completion. A further 100 million doses will be produced by a leading Indian pharmaceutical company Hetero as part of their recent agreement with the Russian Direct Investment Fund. This expanding production and distribution of Sputnik V may hold the potential to flood the global vaccine market in the near future.
#RDIF and @heteroofficial, one of India’s leading generic pharmaceutical companies have agreed to produce in #India over 100 million doses per year of the world’s first registered vaccine against the novel #coronavirus infection – @sputnikvaccine. pic.twitter.com/PPLOiChbR9
— RDIF (@rdif_ru) November 27, 2020
Four leading contenders of COVID-19 vaccines are emerging from Chinese pharmaceutical companies CanSino Biologics Inc., Sinovac, and state-owned Sinopharm. The latter has two vaccines with one already approved for emergency use in the city of Jiaxing; similar to Sinovac’s emergency approval for health care workers in the U.A.E. According to Zheng Zhongwei, head of China’s COVID-19 taskforce, the country has the capacity to produce 600 million doses this year and another billion in 2021. Pioneering four of the world’s leading COVID-19 vaccines, China will play a pivotal role in world immunization.
Following a notion of vaccine diplomacy, China’s President Xi Jinping declared that Chinese vaccines will be made a “global public good.” Delivering to this, China is stepping up to meet much of Latin America and Africa’s vaccine demands. Sinovac already has deals in place that will see 60 million doses delivered to Brazil and another 60 million to Chile, with Morocco also recently ordering 10 million doses of the Sinopharm vaccine.
With limited initial vaccine supplies, wealthier countries are securing stockpiles of vaccines and preventing less affluent nations from receiving the bare minimum of what they need. Total confirmed vaccine purchases are estimated at 7.2 billion doses, half of which are from the EU and five other rich countries despite only accounting for 13% of the world’s population. Making this point even starker, Canada has bought enough doses to vaccinate its entire population five times over whilst “90% of people in 67 low-income countries stand little chance of getting vaccinated against covid-19 in 2021,” according to the British Medical Journal.
Covid-19 Vaccine Doses…
How many have been secured by various countries
Source: Duke University Launch and Scale Speedometer
Analysis by: InShorts pic.twitter.com/W7vv2ykta6— Data Reveals (@DataRvls) December 6, 2020
Whilst it looks like middle and low-income countries are being pushed out by wealthier countries’ bids for vaccines, there may be a saving grace: the COVAX initiative. This global collaboration involves 172 countries and works to provide equitable COVID-19 vaccine access to all countries. COVAX aims to have 2 billion doses of vaccines available by the end of 2021. They hope the equal distribution of this stock will prevent wealthier nations from hoarding vaccines, allowing the initiative to reach its goal of immunizing 20% of every country’s population.
Each vaccine’s distribution requires a unique set of logistical needs, meaning no one delivery method is best for all. For instance, both the Moderna and Pfizer/BioNTech vaccines require storage at incredibly low temperatures of -20°C and -70 °C respectively, whilst the latter only comes in batches of 1,000 doses. As they need expensive supply chains of cold-freezers and storage facilities that few sites have, widespread distribution of these vaccines based on demand is not yet feasible.
On the other hand, vaccines that can be safely stored at more reasonable temperatures will be much easier to distribute. Despite displaying a lower immunity efficiency of 90%, Oxford/AstraZeneca’s vaccine is “fridge-stable” and can be delivered to locations other vaccines cannot reach as it can withstand higher temperatures. Oxford/AstraZeneca have supplied the UK with 4 million doses so far; domestic distribution and vaccination roll-out now hinge on review and approval from the Medicines and Healthcare products Regulatory Agency.
Related Articles: COVID-19 Vaccines: Science is Out to “Getcha”! | COVID-19: One Health Approach
Not even wealthy countries will have enough vaccines for their entire populations at first. Governments will have to decide which groups of people are vaccinated first; not a simple task when all groups – those with the highest risk of exposure, key-workers for essential services and the elderly as the most vulnerable – have convincing reasons to receive it first.
At the recommendation of the Joint Committee on Vaccination and Immunisation (JCVI), the UK has adopted an age-based approach that prioritises the elderly and frontline health and social care workers, as shown below. While this strategy is sound in its reasoning, any prioritisation creates health inequalities. The approach also seems inconsistent in targeting those with increased risk of hospitalisation and death from COVID-19 as it gives no prioritisation to Black, Asian and Minority Ethnic (BAME) groups – which are at greater risk of virus infection and mortality.
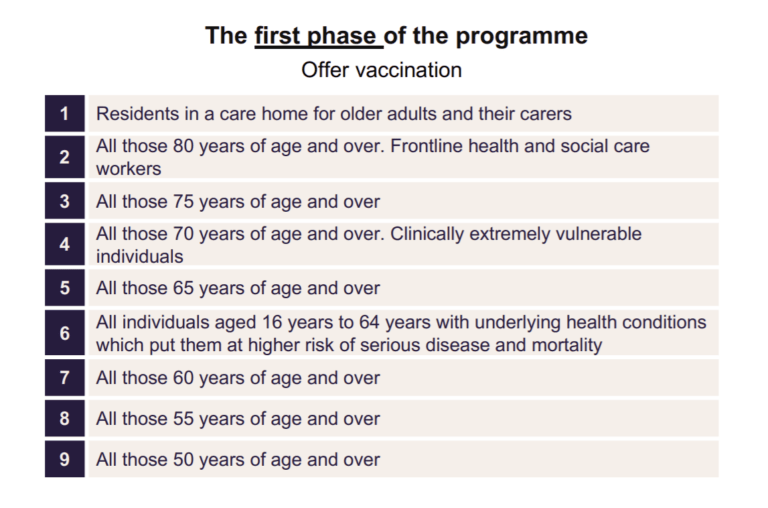
Singling out ethnic minorities may not be the answer as it may inadvertently increase discrimination and ethnic related stigma of the virus. Instead the governments must ensure all ethnic groups have equitable access to vaccines. Failure here – like the UK’s shingles vaccination programme that saw mixed white and black Africans receive 21.8% less vaccine coverage than white groups – would increase the susceptibility of already predisposed groups.
The global reach of the COVID-19 pandemic has been met with an equally global effort to produce vaccines; and finally, they are being delivered. Since governments and companies are unlikely to delay a vaccine that others are already distributing, one vaccine approval is likely to lead to more. A point made clear with Canada’s recent approval of the Pfizer/BioNTech COVID-19 vaccine; implying a high likelihood of a spring-like bloom of vaccine certifications over the coming months, if not weeks. This should lead to a redirection of concerns from when the vaccines will be available to how they will be distributed and to whom they will be given.
Editor’s Note: The opinions expressed here by Impakter.com columnists are their own, not those of Impakter.com. — In the Featured Photo: Army Spc. Angel Laureano holds a vial of the COVID-19 vaccine, Walter Reed National Military Medical Center, Bethesda, Md., Dec. 14, 2020. Featured Photo Credit: U.S. Secretary of Defense/Lisa Ferdinando.