No magic bullet is in sight yet, but three vaccines for particular skin and lung cancer types have advanced to the last phase of clinical trials
A cure for cancer — which is second only to cardiovascular diseases in its contribution to the global burden of disease — has long been a dream.
While no magic bullet is yet in sight, three vaccines for particular skin and lung cancer types have advanced to the last stage of clinical trials in recent months.
If successful, these cancer vaccines should be available to patients in the next three to 11 years. Unlike vaccines which prevent diseases, these aim to cure them or prevent relapses.
Cancer in every person is different because the cells in every cancerous tumour have different sets of genetic mutations. Recognising this, two of the vaccines are personalised and tailor-made for each patient. Oncologists working with pharmaceutical companies have developed these individualised neoantigen therapies.
A vaccine typically works by training the immune cells of our body to recognise antigens — proteins from pathogens, such as viruses — against future attacks by the pathogen.
In cancer, however, there is no external pathogen. The cells of a cancerous tumour undergo continuous mutations, some of which help them to grow much faster than normal cells while some others help them evade the body’s natural immune system. The mutated proteins in cancerous cells are called “neoantigens.”
In individualised neoantigen therapy, the gene sequence of the tumour and normal blood cells are compared to identify neoantigens from each patient, and then a subset of neoantigens are chosen that are most likely to induce an immune response. The vaccine for an individual patient targets this chosen subset of neoantigens.
These vaccines, jointly developed by pharma giants Moderna and Merck, have been shown in trials conducted so far to be significantly more effective in combination with immunotherapy than immunotherapy alone in preventing both the relapse of melanoma — a type of skin cancer — and non-small cell lung cancer after the tumours had been surgically removed.
Following these promising results in phase II clinical trials, the vaccines are now being tested on a larger group of patients in phase III trials. The studies are expected to be complete by 2030 for melanoma and 2035 for lung cancer.
The Moderna-Merck cancer vaccine may not be the first to reach the market. The French company OSE Immunotherapeutics published positive results last September from phase III clinical trials of a vaccine using a different approach for advanced non-small cell lung cancer. Its vaccine, Tedopi, is scheduled to start confirmatory trials — which are the last step before regulatory approval — later this year and may be available by 2027.
Vaccines for pancreatic cancer being developed by BioNTech and Genentech, and for colon cancer by Gritstone, are also showing promising results in the early phases of clinical trials. Like the vaccines being developed by Moderna and Merck, these too are individualised neoantigen therapies based on messenger RNA (mRNA).
There is another kind of RNA therapy also under development that uses small interfering RNA (siRNA) and microRNA (miRNA). Since 2018, six siRNA-based therapies have been approved by the US Food and Drug Administration for the treatment of neural, skin, heart and renal diseases. Several more siRNA drugs are at various clinical trial stages for different types of cancer and a diverse range of other diseases.
Within cells, there are two kinds of nucleic acid molecules that contain coded information vital to life: DNA and RNA. While DNA contains genetic information, mRNA — one among the different types of RNA — carries the codes for the proteins. In addition, there are also non-coding RNA, some of which are functionally important. siRNA and miRNA are examples of such non-coding RNA.
Related Articles: Cancer Hits the Young More Than Ever | New ‘Self-Amplifying’ RNA-Based Vaccine: Progress or Possible Problems? | Anti-Vaxxers and Vaccine Hesitancy: A Cottage Industry Gone Big Time | WHO Approves Malaria Vaccine in Scientific Breakthrough
The RNA vaccine for an individualised neoantigen therapy is a cocktail of mRNA carrying the codes for neoantigens — the mutated fingerprint proteins in cancerous cells. For the Moderna-Merck study, scientists identified 34 neoantigens per patient. They delivered the corresponding mRNA vaccine cocktail packed in lipid nanoparticles, just like the mRNA vaccines for COVID-19 developed by Moderna and Pfizer-BioNTech.
When the vaccine is delivered after removing the tumour, it trains the immune system to recognise neoantigens and fight back against the cancer returning. Usually, the body’s natural immune system corrects mutations and prevents us from having cancers. However, in some cases this natural immune response is insufficient, leading to tumour growth. In individualised neoantigen therapy, these mutations in the tumour cells are used for vaccine development and for training the immune system to fight back against relapse after removal of the tumour.
Recent advances in artificial intelligence are helping identify potential neoantigens and manage personalised therapies. Firstly, gene sequencing of tumours and normal blood cells of a patient and their comparison produces a huge amount of data. AI is used to find the genetic mutations of the patient’s cancer in such ‘big data’. Moreover, individualised therapy requires timely production and delivery of vaccines that are different for each patient. AI is also useful in the management of such data.
The individualised nature of the treatment is probably why it has been more effective in trials than previous, unsuccessful RNA vaccine candidates. However, this personalisation is also likely to raise challenges for the timely and cost-effective delivery of treatment to populations around the world.
The siRNA and miRNA treatments work in a way opposite to mRNA. While each mRNA in a vaccine carries the code for producing a protein from a pathogen (antigen) or tumour (neoantigen) to train our immune systems against future attacks by the pathogen or tumour, siRNA directly targets the mRNA of the antigen or neoantigen and terminates the production of the protein it codes. Thus, the effect of a siRNA is more direct and immediate (like a drug), rather than a protection against future attacks (like a vaccine).
Discovered at the turn of this millennium, siRNA-based therapeutics attracted immediate attention, but their initial success was limited due to their inherent low stability, difficulties in delivering them to desired locations, and rapid clearance from the bloodstream. However, in recent years, siRNA therapies have been boosted through chemical modifications that have increased their stability and ability to be delivered to specific locations such as tumours, and improved delivery systems such as lipid nanoparticle encasings.
These improvements led to recent successes in FDA approvals of siRNA-based therapies and further promising reports of advances in the treatment of diseases including a type of liver cancer.
** **
This article was originally published by 360info™.
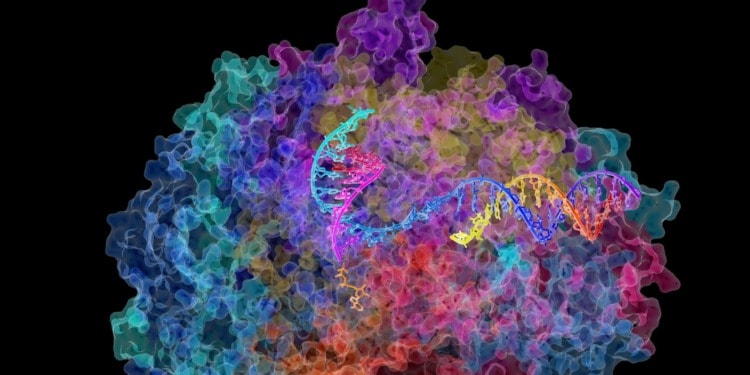
Editor’s Note: The opinions expressed here by the authors are their own, not those of Impakter.com — In the Cover Photo: RNA polymerase II. This is the enzyme in mammalian cells that catalyses the transcription of DNA into messenger RNA, the molecule that in turn dictates the order of amino acids in proteins. Cover Photo Credit: David Bushnell, Ken Westover and Roger Kornberg, Stanford University / National Institutes of Health (NIH) via Flickr.